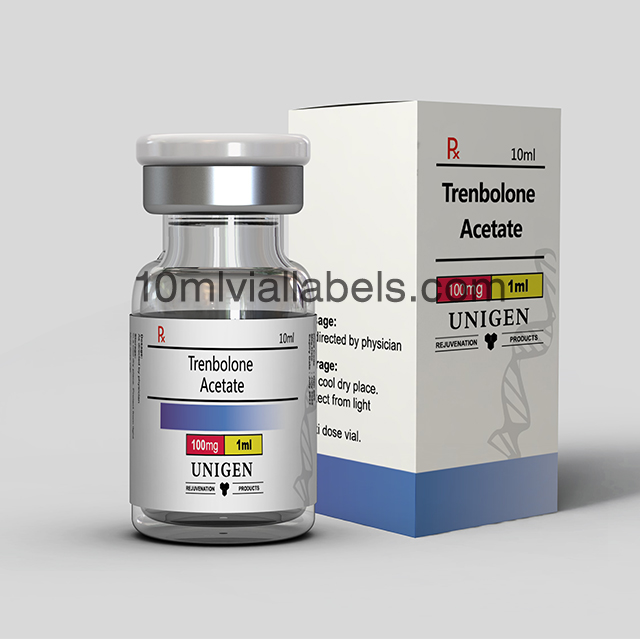
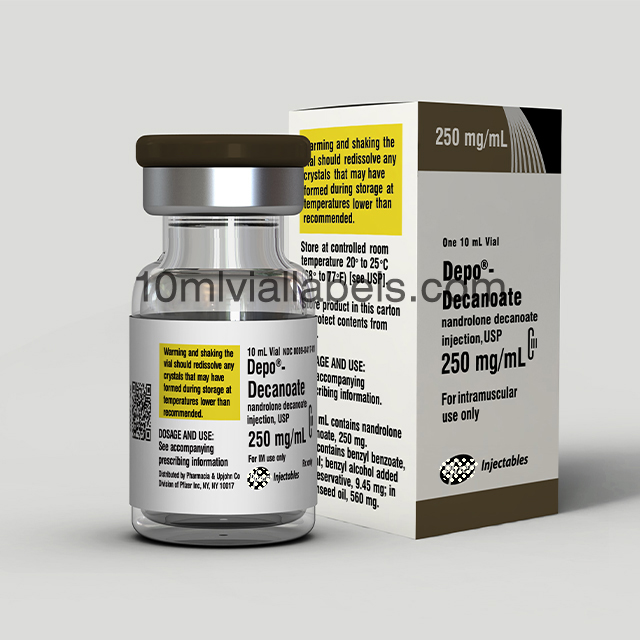
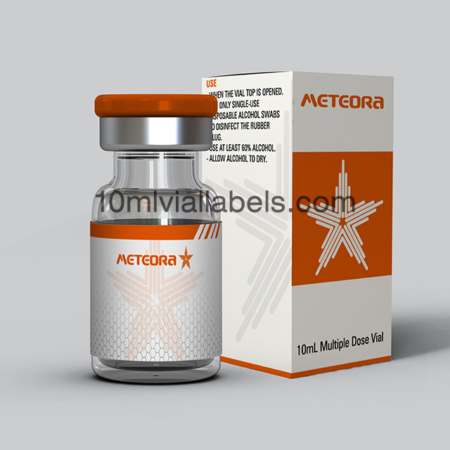
Glass bottle vial lables for phatmacy steroids
Pharmacy steroid glass bottle labels are labels specifically designed for use on glass vials or bottles that contain steroids in a pharmacy or medical setting.
They provide essential information about the contents of the vial, including the name of the steroid, its concentration, dosage instructions, and any other relevant details. This helps pharmacists and healthcare professionals accurately identify and dispense the medication.
Labels may include safety warnings, such as storage instructions, expiration dates, and cautionary statements to ensure the safe handling and use of steroids.
Pharmacy steroid bottle labels often need to comply with regulatory requirements and standards set by health authorities to ensure patient safety and proper medication management.
They can include barcodes or serial numbers to facilitate accurate tracking and tracing of the medication throughout the supply chain, from manufacturing to distribution and patient administration.
In some cases, labels may also incorporate branding elements, such as the pharmacy’s logo or name, to create a professional and consistent look for the pharmacy’s products.To prevent counterfeiting and ensure the authenticity of pharmaceutical products, some labels may incorporate security features like holograms or tamper-evident seals.
When designing and printing pharmacy steroid glass bottle labels, it’s crucial to adhere to industry regulations and standards to ensure patient safety and regulatory compliance. These labels play a critical role in medication management and patient care within a pharmacy or healthcare facility.

What content should I put on the vial labels design?
A pharmaceutical packaging label serves as a crucial communication tool for medication, providing essential information to healthcare professionals and patients. Here is a list of key information that is typically required on pharmaceutical packaging labels:
Drug Name: The name of the medication, including both the brand (if applicable) and generic name.
Strength or Dosage: The concentration or strength of the medication, indicating the amount of the active ingredient per unit.
Dosage Form: Whether the medication is a tablet, capsule, liquid, cream, injection, etc.
Usage Instructions: Clear and concise dosage instructions, including the frequency and method of administration.
Indications: The specific medical conditions or symptoms the medication is intended to treat.
Contraindications: Any situations or conditions in which the medication should not be used.
Warnings and Precautions: Important safety information, including potential side effects, drug interactions, and special precautions.
Expiration Date: The date beyond which the medication is no longer guaranteed to be effective.
Lot Number: A unique identifier for the batch or lot of medication, which aids in tracking and tracing.
Barcodes: Barcodes, including a National Drug Code (NDC) or other tracking codes, to assist with inventory management and patient safety.
Storage Instructions: Guidance on how the medication should be stored (e.g., room temperature, refrigeration, protection from light).
Manufacturer Information: The name and contact information of the pharmaceutical manufacturer or distributor.
Pharmacy Information: Details about the dispensing pharmacy, including its name, address, and contact information.
Patient Information: Space for adding patient-specific details, such as the patient’s name, prescription number, and directions for use.
Generic Equivalents: Information about whether a generic version of the medication is available.
Regulatory Information: Compliance with regulatory requirements, including FDA or other relevant agency approvals and certifications.
Language: The label should be in the appropriate language(s) for the region where it will be distributed.
Special Handling Instructions: Any specific instructions for healthcare providers or patients on how to handle the medication (e.g., for refrigerated items).
Branding: If applicable, branding elements such as the pharmaceutical company’s logo or trademark.
Tamper-Evident Features: Security features to ensure the integrity of the medication package.
Additional Information: Any additional information required by local regulations or specific to the medication.
Pharmaceutical packaging labels play a vital role in patient safety, medication adherence, and regulatory compliance. Ensuring that all necessary information is accurately and clearly presented on the label is critical in the pharmaceutical industry.

Order Process

Payment

Logistics and Transportation




Reviews
There are no reviews yet.