Use orange color in the steroid vial labels and vial boxes
Using the color orange in pharmaceuticals can serve various purposes, especially in the latest anabolic steroids ares, including branding, product identification, and safety measures. Here are some common uses of the color orange in the pharmaceutical industry:
Safety Packaging: Orange is often used for the packaging of prescription medications to help differentiate them from over-the-counter (OTC) drugs. This color coding helps pharmacists and consumers easily identify the more potent and potentially riskier prescription medications.
Dosage and Strength Identification: Orange can be used on medication vial labels, tablets, or capsules to indicate a specific dosage or strength. This aids patients and healthcare professionals in quickly recognizing the correct medication and its potency.
Branding: Pharmaceutical companies may incorporate the color orange into their branding and packaging design to create a distinctive and recognizable image for their products. This can help with brand loyalty and consumer trust.
Safety Warnings: Some steroids, especially those with potentially serious side effects or interactions, may use orange labeling or warning labels to draw attention to the importance of following dosing instructions and consulting a healthcare provider.
Flavor and Appearance: Orange flavoring and coloring are often used in liquid medications to make them more palatable, especially for pediatric patients. This can encourage compliance with medication regimens.
Marketing and Allergen Warnings: Orange may also be used in marketing materials and vial labels to highlight information related to allergens or special dietary considerations for certain medications.
It’s important to note that the use of colors in steorids packaging should adhere to regulatory guidelines and standards to ensure safety, clarity, and effectiveness in communicating important information to patients and healthcare professionals. Additionally, colors should not be used in a way that could be confusing or misleading regarding a medication’s purpose or ingredients.
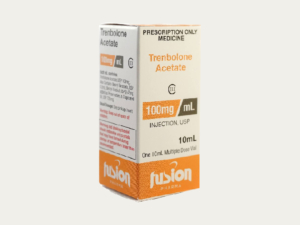
What is the entire 10ml injectable steroids packaging?
A 10ml injection of pharmaceutical packaging typically might inlcuding as follow:
Active Ingredient: This is the primary therapeutic substance that provides the intended medicinal effect. It can be a drug, vaccine, hormone, or other bioactive compound.
Excipients: Excipients are inactive ingredients or additives used to formulate the injection. They serve various purposes, such as preserving the stability of the active ingredient, adjusting the pH level, enhancing solubility, or aiding in the manufacturing process. Common excipients include:
- Water for Injection (WFI): Purified water used as a solvent.
- Preservatives: Chemicals added to prevent microbial growth in multi-dose vials.
- Buffers: Substances used to maintain the injection’s pH at a suitable level.
- Antioxidants: Compounds that protect the active ingredient from oxidation.
- Tonicity Adjusters: Substances used to adjust the injection’s osmolarity to match that of bodily fluids.
- Stabilizers: Compounds that help maintain the stability of the active ingredient over time.
- Surfactants: Surface-active agents that improve the dispersion of substances in the injection.
- Solvents: Additional solvents may be used to dissolve the active ingredient if it’s not water-soluble.
Container: The pharmaceutical injection is typically stored in a vial or ampoule made of glass or plastic. The container must be sterile to prevent contamination.
Closure: The closure is the seal on the vial or ampoule, which is usually made of rubber or elastomeric material. It maintains the sterility of the contents and allows for the withdrawal of the solution with a needle and syringe.
Labeling: The label on the injection provides essential information, including the medication’s name, concentration, dosage instructions, expiration date, lot number, and manufacturer information.
Instructions for Use: Depending on the medication, there may be specific instructions for reconstitution (if required) and administration.
Safety Features: Some pharmaceutical injections, especially those prone to abuse or misuse, may incorporate safety features such as tamper-evident caps or anti-counterfeiting measures.
The specific composition of a 10ml injection pharmaceutical will vary depending on the medication’s purpose and formulation. It’s essential to follow the instructions provided by a healthcare professional or on the medication’s label when using pharmaceutical injections, as improper use can lead to adverse effects or reduced efficacy.

Order Process

Payment

Logistics and Transportation





Reviews
There are no reviews yet.