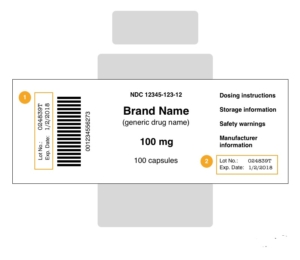
Why do you need the lot number, mfg date, and exp date on your vial labels and vial box design?
In the pharmaceutical industry, the inclusion of lot numbers, manufacturing dates, and expiration dates on vial labels and packaging plays a pivotal role in ensuring the safety and quality of the products. These essential pieces of information provide critical insights into the origin, production, and validity of pharmaceuticals, enabling effective traceability and regulatory compliance.
Ensuring Traceability and Quality Control
Including the lot number, manufacturing date, and expiration date on vial labels and packaging allows for better traceability throughout the supply chain. Each lot number represents a specific batch of products, enabling manufacturers to track and recall specific vials if necessary. In the event of a quality issue or product recall, this information helps identify affected batches promptly, ensuring targeted action to address the problem.
Furthermore, the manufacturing date and expiration date provide critical insights into the shelf life of the product. By clearly indicating the manufacturing date, healthcare professionals and consumers can determine the age of the vial, allowing for proper stock rotation and reducing the risk of using expired products.
Enhancing Patient Safety
The inclusion of lot numbers, manufacturing dates, and expiration dates on vial labels and packaging significantly contributes to patient safety. With this information readily available, healthcare professionals can verify the authenticity and integrity of the product before administration. In case of adverse events or issues related to specific batches, lot numbers assist in identifying the source and facilitating timely intervention.
Additionally, expiration dates ensure that medications are used within their intended timeframe. Expired drugs may lose potency or even become harmful, potentially compromising patient outcomes. By prominently displaying expiration dates, healthcare providers and patients can confidently make informed decisions regarding the use of medications.
Regulatory Compliance and Auditing
The pharmaceutical industry is subject to strict regulations and quality standards to safeguard public health. Including lot numbers, manufacturing dates, and expiration dates on vial labels and packaging is an essential part of regulatory compliance. Health authorities and auditors can easily verify that manufacturers are adhering to Good Manufacturing Practices (GMP) and are following proper quality control procedures.
These details also aid in investigations and audits, providing a comprehensive record of product information and ensuring transparency in the manufacturing process. By complying with regulatory requirements, pharmaceutical companies demonstrate their commitment to quality and patient safety.
Lot numbers, manufacturing dates, and expiration dates play a vital role in pharmaceutical packaging, providing traceability, enhancing patient safety, and ensuring regulatory compliance. By including this information on vial labels and packaging, manufacturers contribute to the overall quality assurance of pharmaceutical products, supporting patient well-being and maintaining industry standards.
How do you occupy the steroid market with your vial box design?
To effectively occupy the steroid market with your vial box design, it is crucial to focus on several key aspects that can set your product apart from competitors. Here are some strategies to consider:
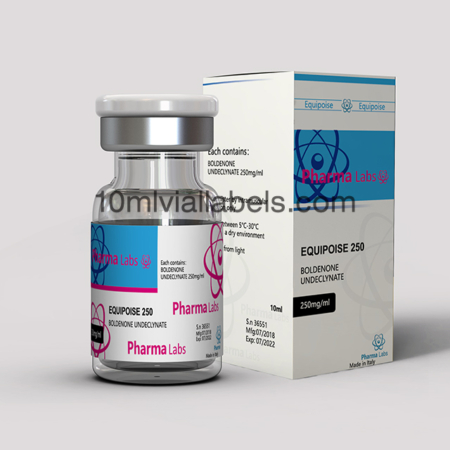
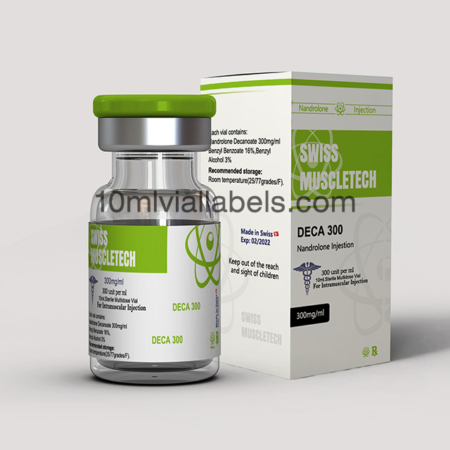
Functional and User-Friendly Design: Create a vial box design that is easy to use and provides convenience to both healthcare professionals and patients. Consider features such as easy-open mechanisms, clear labeling, and ergonomic designs that facilitate efficient handling and administration of the steroids.
Secure and Tamper-Proof Packaging: Develop a vial box design that incorporates tamper-evident features to ensure product integrity and prevent unauthorized access. This can include seals, holographic stickers, or other innovative tamper-proof technologies that instill confidence in the safety and authenticity of the product.

Informative and Compliant Labeling: Pay close attention to the labeling on the vial box, ensuring compliance with regulatory requirements and industry standards. Clearly display essential information such as the steroid name, dosage, lot number, manufacturing date, and expiration date. Use legible fonts, appropriate colors, and prominent placement to make the information easily readable and accessible.
Differentiate through Branding: Create a distinct and recognizable brand identity for your steroid product. This can be achieved through thoughtful branding elements, such as a unique logo, color scheme, and typography. Consistently apply these branding elements across the vial box design, packaging materials, and marketing collateral to establish a strong brand presence in the market.
Aesthetically Pleasing Design: Invest in an aesthetically appealing vial box design that catches the attention of potential customers. Consider engaging graphic designs, visually appealing illustrations, or even collaboration with talented artists to create visually stunning packaging that stands out on pharmacy shelves and attracts consumers.
Emphasize Safety and Quality Assurance: Highlight safety features and quality assurance measures in your vial box design. Incorporate symbols or graphics that represent compliance with industry standards, such as GMP (Good Manufacturing Practices) or ISO certifications. Reinforce the message that your product prioritizes patient safety and follows stringent quality control protocols.

Effective Marketing and Promotion: Develop a comprehensive marketing strategy to promote your vial box design in the steroid market. Utilize various channels, including online platforms, medical conferences, and collaborations with healthcare professionals, to raise awareness about your product’s unique features, benefits, and advantages. Leverage social media, professional networks, and targeted advertising to reach your target audience effectively.
By combining these strategies in your vial box design and marketing efforts, you can position your steroid product as a standout choice in the market, gaining a competitive edge and capturing a significant share of the steroid market.
Order Process

Payment

Logistics and Transportation




Reviews
There are no reviews yet.